What factors accelerate the deactivation of denitrification catalysts?
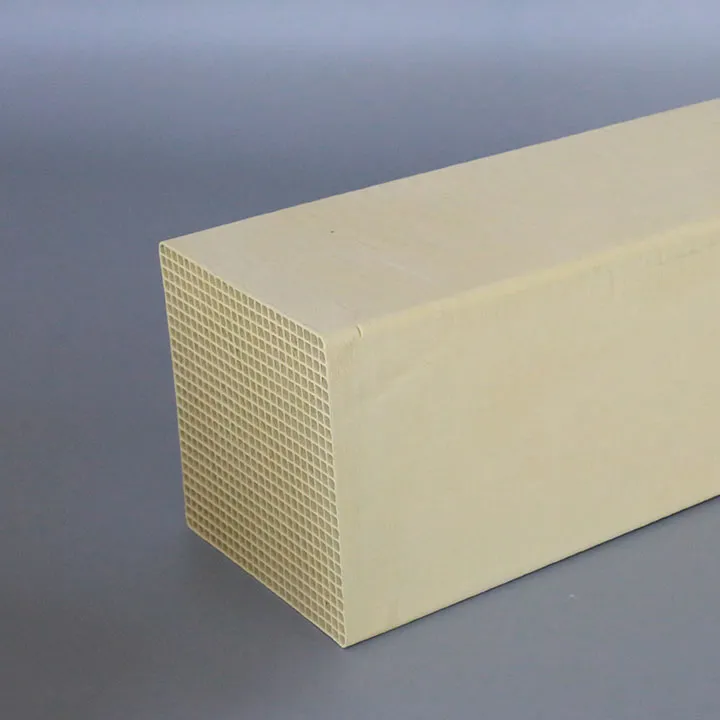
As an important means to reduce nitrogen oxide (NOx) emissions, denitrification catalysts play a key role in industrial production. As the use time increases, the activity of the catalyst gradually decreases, which is the so-called catalyst deactivation phenomenon. Deactivation will not only affect denitrification efficiency, but also cause corporate emissions to fail to meet standards, bringing dual environmental and economic pressures.
1. Effect of high temperature on catalysts
Temperature is one of the important factors affecting the activity of denitration catalysts. During the SCR (selective catalytic reduction) reaction process, the catalyst usually works at a higher temperature to ensure the smooth progress of the reaction. However, active ingredients on the surface of ultra-high temperature SCR denitrification catalyst, such as vanadium (V), tungsten (W), etc., may migrate or be lost, thereby reducing its catalytic activity.
In addition, high temperature will accelerate the sintering of the pores on the catalyst surface, reducing the specific surface area of the catalyst and reducing the number of active sites. In this case, the activity of the catalyst decreases significantly, thus accelerating the deactivation process. In order to extend the service life of the catalyst, companies should strictly control the reaction temperature and avoid long-term overload operation.
2. Dangers of heavy metal pollution
Heavy metal pollution is another major reason for accelerated denitration catalyst deactivation. Heavy metals such as lead (Pb), mercury (Hg), arsenic (As), etc. are common in industrial waste gases. Once these metal ions come into contact with the catalyst, they may react with the active centers on the catalyst surface, thereby poisoning the catalyst.
This poisoning effect usually manifests as the active components on the catalyst surface being occupied by heavy metals, which hinders the contact between NOx and the reducing agent, resulting in a reduction in reaction efficiency. To prevent heavy metal pollution, companies should take effective waste gas pretreatment measures, such as using filtering devices or chemical adsorbents to remove heavy metals before the waste gas enters the denitrification reactor.

3. Effect of sulfides
Sulfides, especially sulfur dioxide (SO2), easily react with active components in the catalyst to form sulfate or sulfuric acid at high temperatures, which then cover the catalyst surface, causing the surface active sites to be obscured. This situation will significantly reduce the activity of the catalyst, causing it to lose its catalytic ability.
In addition, the generation of sulfate may also cause changes in the pore structure of the catalyst, affect the gas permeability, and accelerate the deactivation of the catalyst. Therefore, in the process of using denitration catalysts, it is crucial to control the concentration of sulfide. Enterprises should regularly monitor the sulfide content in exhaust gas and adjust denitrification process parameters as needed.
4. Deposition of dust particles
Industrial waste gas often contains a large number of dust particles. After these particles are deposited on the surface of the catalyst, they will block the pores of the catalyst, affect the contact between the reactants and the catalyst, and reduce the catalytic efficiency. Long-term accumulation of dust may even form solid deposits, further accelerating catalyst deactivation.
In order to prevent the impact of dust on the catalyst, companies can add pre-dust removal equipment, such as electrostatic precipitators or bag dust collectors, to the exhaust gas treatment system to minimize the concentration of dust entering the catalyst reactor. In addition, regular cleaning or replacement of catalysts is also an effective measure to maintain catalyst activity.

5. Effect of oxides
Certain oxides such as alkali metal oxides (such as Na2O, K2O) and alkaline earth metal oxides (such as CaO, MgO) will irreversibly react with the active components of the catalyst to form stable compounds. These compounds are often inactive and cannot continue to participate in the denitration reaction, resulting in reduced catalyst activity.
These oxides often originate from impurities in the fuel or by-products produced during reactions. In order to reduce the impact of oxides on the catalyst, companies should choose fuels with higher purity and set up appropriate filtration or adsorption devices in the denitrification system to remove harmful oxides.
6. Comprehensive factors of catalyst poisoning
Catalyst poisoning is a complex problem that causes denitration catalyst deactivation. In addition to the heavy metals, sulfides and other factors mentioned above, it also includes toxic substances such as chloride and phosphide. Once these substances enter the denitration system, they may strongly combine with the active center of the catalyst to form stable poisoning substances, thereby causing the catalyst to lose activity.
In response to the problem of catalyst poisoning, companies should adopt a variety of technical means to comprehensively respond, such as reducing the generation of toxic substances at the source, strengthening exhaust gas pretreatment, selecting catalyst materials with excellent toxicity resistance, etc. In addition, regular catalyst activity testing and timely detection and treatment of poisoning problems will also help extend the service life of the catalyst.
7. The catalyst is used for too long
Even under the most ideal conditions, catalyst activity gradually decreases over time. This is because the catalyst will be affected by the cumulative effects of many factors during long-term operation, such as thermal aging, mechanical wear, chemical corrosion, etc.
Therefore, enterprises should replace or regenerate the catalyst within an appropriate time based on the service life of the catalyst. Regenerated catalysts can restore part of their activity through heat treatment, chemical washing and other methods, thereby extending their service life and reducing the company’s operating costs.

Conclusion
The deactivation of denitration catalyst is an inevitable but controllable process. By understanding and controlling various factors that affect catalyst deactivation, companies can effectively extend the service life of the catalyst, improve denitration efficiency, and ensure the environmental compliance of the production process. In actual operations, companies should comprehensively consider various influencing factors and adopt multi-level and multi-dimensional technical measures to achieve the best operating status of the denitrification system.
It is hoped that this article can provide valuable reference for technicians engaged in the use and maintenance of denitration catalysts, help them better cope with the challenges caused by catalyst deactivation, and contribute to environmental protection.