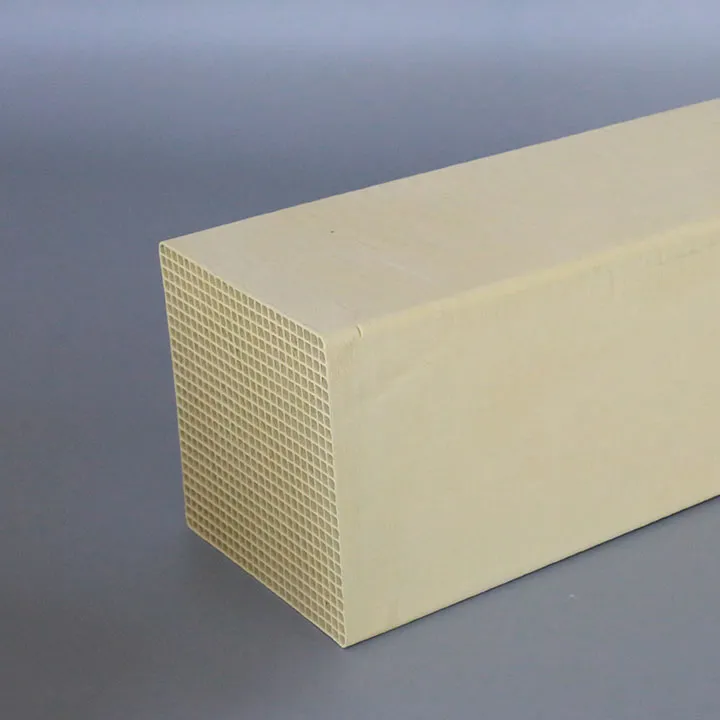
Bloqueio de microporos em catalisadores de desnitrificação: Análise de causas e soluções de otimização
Na operação e uso reais, embora a superfície do catalisador de desnitrificação seja frequentemente mantida limpa, os microporos internos são bloqueados de tempos em tempos. Esse problema incomoda muitas empresas e afeta diretamente a eficiência geral e a vida útil do sistema de desnitrificação. Então, o que exatamente causa essa situação? Como resolver esses problemas para garantir a operação eficiente e de longo prazo do catalisador de desnitrificação? Este artigo analisará isso em detalhes, analisará as causas do problema das perspectivas de penetração de partículas de poeira, deposição de sulfato e acúmulo de cinzas volantes e proporá soluções de otimização para lidar com o bloqueio, ajudando as empresas a estender a vida útil do catalisador e melhorar a eficiência do sistema de desnitrificação.
Sete razões para o bloqueio de microporos em catalisadores de desnitrificação
1. Penetração de poeira e partículas: perigos ocultos invisíveis Durante a reação de desnitrificação, embora a superfície externa do catalisador possa permanecer limpa, algumas pequenas poeiras e partículas podem penetrar na superfície e entrar na estrutura microporosa dentro do catalisador. Essas partículas finas são difíceis de remover a tempo devido ao seu pequeno tamanho. Com o tempo, elas se acumularão gradualmente dentro dos microporos, resultando em uma redução na área de superfície efetiva do catalisador, afetando assim a eficiência da reação de desnitrificação. Comparado com a superfície, o interior dos microporos é mais difícil de limpar, então esse tipo de bloqueio é frequentemente difícil de detectar e se torna um perigo oculto potencial.
2. Deposição de sulfato: um assassino silencioso No processo de uso de combustíveis contendo enxofre, o dióxido de enxofre (SO₂) gerado pela combustão reage com outras substâncias no catalisador para formar sulfatos. Esses sulfatos geralmente se depositam profundamente nos microporos do catalisador, fazendo com que os microporos gradualmente obstruam. Devido à limpeza do fluxo de ar e às altas temperaturas, a superfície externa geralmente não é fácil de depositar, então o catalisador ainda pode parecer intacto, mas o interior dos microporos foi lentamente erodido pelos sulfatos. Esse processo de obstrução silenciosa reduzirá gradualmente a atividade do catalisador e o efeito geral de desnitrificação.
3. Acúmulo de cinzas volantes: acúmulo silencioso Em ambientes industriais, especialmente em usinas de energia a carvão, o ar frequentemente carrega uma grande quantidade de cinzas volantes e partículas ultrafinas. Devido ao seu pequeno tamanho, essas partículas podem facilmente penetrar nos poros da superfície do catalisador, entrar na estrutura microporosa e gradualmente se acomodar. Esse fenômeno não mostra imediatamente um problema, mas com o tempo, o acúmulo de cinzas volantes nos microporos reduzirá gradualmente a área de reação do catalisador, reduzindo assim a eficiência da desnitrificação. Nesse ponto, a limpeza da superfície não reflete a verdadeira situação dentro do catalisador, tornando o problema mais difícil de detectar.
4. Envelhecimento do catalisador: uma lei natural inevitável Durante o uso a longo prazo do catalisador, a estrutura microporosa inevitavelmente envelhecerá, o tamanho dos poros diminuirá gradualmente e o espaço interno ficará menor. Esse fenômeno de envelhecimento não apenas facilita o acúmulo de partículas nos microporos, mas também leva ao aumento do bloqueio dos microporos. Mesmo que não haja bloqueio óbvio na superfície externa, as mudanças estruturais internas causadas pelo envelhecimento afetarão o desempenho geral do catalisador. Isso exige que monitoremos regularmente a condição do catalisador para evitar o impacto a longo prazo dos problemas de envelhecimento na eficiência da desnitrificação.
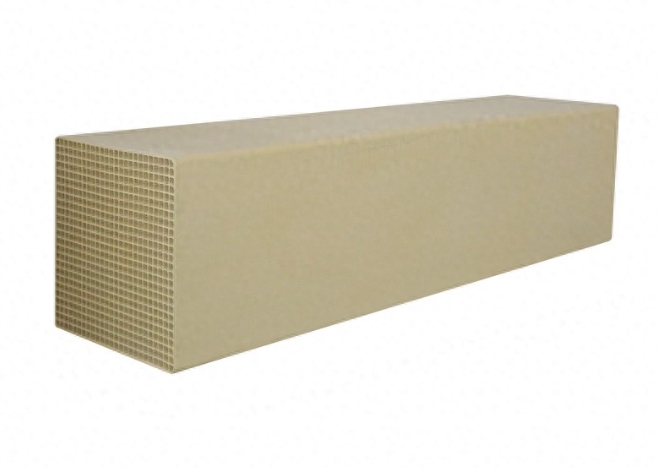
5. Acúmulo de subprodutos de reação incompletos: produtos inesperados Na reação de desnitrificação, a amônia (NH₃) reage com óxidos de nitrogênio (NOx) como um agente redutor para gerar nitrogênio (N₂) e vapor de água. No entanto, sob certas condições, essa reação pode não ser completa, produzindo alguns subprodutos, como sais de amônio (NH₄HSO₄). Esses subprodutos são facilmente depositados nos microporos do catalisador, causando bloqueio de poros e afetando a atividade do catalisador. Especialmente quando as condições de reação não são ideais, o acúmulo de subprodutos pode ser mais sério, afetando ainda mais o desempenho do catalisador. Neste momento, embora a aparência do catalisador possa não mudar muito, os microporos internos foram silenciosamente ocupados por subprodutos.
6. A influência do vapor de água e das flutuações de temperatura: fatores ambientais que não podem ser ignorados Durante a operação do sistema de desnitrificação, se houver vapor de água excessivo ou a temperatura operacional flutuar frequentemente, o vapor de água pode se combinar com o gás de reação para formar cristais ou outras substâncias sólidas nos microporos do catalisador. Esses cristais ou substâncias sólidas não podem ser facilmente descarregados, bloqueando gradualmente os microporos e afetando a atividade do catalisador. Especialmente em um ambiente com temperatura instável, mudanças frequentes no vapor de água e na temperatura acelerarão a formação de bloqueios. Embora a superfície externa ainda seja lisa, os microporos internos foram seriamente afetados, e a eficiência da reação de desnitrificação é bastante reduzida.
7. Efeito de limpeza irregular: potencial ponto cego de limpeza A maioria dos sistemas de desnitrificação é equipada com dispositivos de limpeza para evitar que partículas de poeira se depositem. No entanto, se o sistema de limpeza não for projetado corretamente ou o efeito de limpeza for irregular, a superfície do catalisador pode permanecer relativamente limpa, mas o acúmulo de poeira nos microporos internos não é completamente removido. Essa situação fará com que o problema de bloqueio dos microporos se intensifique gradualmente, afetando a eficiência geral da reação do catalisador. A negligência a longo prazo da uniformidade do efeito de limpeza encurtará muito a vida útil do catalisador.
Como resolver o problema de bloqueio de microporos?
Agora que entendemos as principais razões para o bloqueio de microporos, como podemos resolver esse problema de forma eficaz? Aqui estão algumas sugestões para ajudar as empresas a manter melhor o desempenho de catalisadores de desnitrificação nas operações diárias:
1. Limpeza e manutenção regulares Limpe e faça a manutenção do catalisador regularmente, preste atenção especial ao efeito de limpeza dos microporos internos, certifique-se de que o dispositivo de limpeza possa cobrir todas as partes do catalisador e evite o problema de limpeza local incompleta.
2. Monitorar as condições de reação Monitoramento em tempo real das condições operacionais do sistema de desnitrificação, especialmente temperatura e teor de vapor de água, para garantir que o sistema opere em condições ideais e reduzir a geração e deposição de subprodutos.
3. Otimize o combustível e as condições de reação Escolha combustível com baixo teor de enxofre o máximo possível para reduzir a deposição de sulfato. Ao mesmo tempo, otimize as condições de reação de amônia e NOx para evitar problemas de subprodutos causados por reações incompletas.
4. Regeneração do catalisador Para catalisadores que ficam obstruídos após um período de uso, você pode considerar tratá-los por meio da tecnologia de regeneração para restaurar a estrutura microporosa e a atividade do catalisador e prolongar sua vida útil.
5. Verifique regularmente o status do catalisador. Use equipamento de teste especial para verificar regularmente o status do catalisador, especialmente a permeabilidade dos microporos. Por meio de métodos de monitoramento científico, problemas potenciais podem ser descobertos e resolvidos em tempo hábil para evitar que o bloqueio afete o desempenho do sistema.
Conclusão
O bloqueio de microporos é um problema comum no uso a longo prazo de catalisadores de desnitrificação. Embora a superfície possa permanecer limpa, o bloqueio interno está se formando gradualmente. Ao entender a influência de fatores como poeira, cinzas volantes e deposição de sulfato, e tomar medidas eficazes de limpeza, monitoramento e otimização, as empresas podem melhorar muito a eficiência do uso de catalisadores de desnitrificação e garantir a operação estável a longo prazo do sistema. O gerenciamento inteligente e a manutenção científica ajudarão as empresas a permanecerem firmes no caminho da proteção ambiental.