اصول و انتخاب کاتالیزورهای نیترات زدایی: تجزیه و تحلیل عمیق و راهنمای عملی
1. اصل کاتالیزور DeNOx
کاتالیزور DeNOx عمدتاً برای کاتالیز کاهش NOx به نیتروژن (N2) و آب (H2O) استفاده می شود، فرآیندی که کاهش کاتالیستی انتخابی (SCR) نامیده می شود. اصل اساسی آن شامل مراحل کلیدی زیر است:
تزریق احیاکننده: احیا کننده های رایج مورد استفاده شامل آمونیاک (NH3) و اوره (CO(NH2)2) هستند. این احیا کننده ها به طور یکنواخت به گاز دودکش حاوی NOx به روش خاصی تزریق می شوند.
اختلاط یکنواخت: احیاکننده تزریق شده باید به طور کامل با NOx موجود در گاز دودکش مخلوط شود تا از کارایی و اثر واکنش کاتالیزوری بعدی اطمینان حاصل شود.
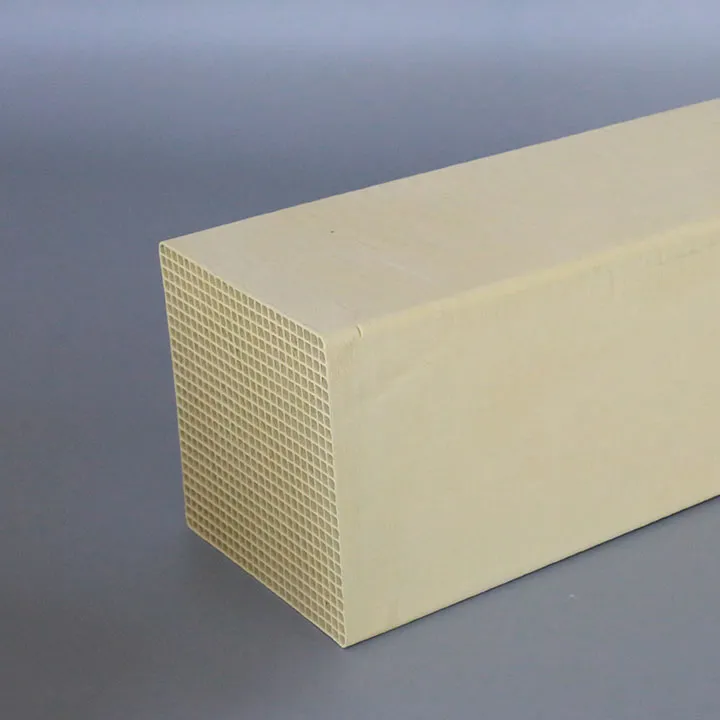
واکنش کاتالیزوری: گاز دودکش مخلوط و ماده احیا کننده از طریق جریان می یابد کاتالیزور DeNOx لایه. تحت عمل کاتالیزور، NOx با احیا کننده واکنش می دهد و به N2 و H2O کاهش می یابد. کاتالیزور معمولاً از موادی مانند تیتانات وانادیم، اسید تنگستیک یا مولیبدات ساخته میشود که میتواند به طور موثری واکنش را تقویت کند و دما و انرژی مورد نیاز برای واکنش را کاهش دهد.
معادلات واکنش: معادلات اصلی واکنش شیمیایی به شرح زیر است:
4NO+4NH3+O2→4N2+6H2O4NO+4NH3+O2→4N2+6H2O
2NO2+4NH3+O2→3N2+6H2O2NO2+4NH3+O2→3N2+6H2O
این معادلات نشان می دهد که NOx تحت تأثیر کاتالیزورها و احیاکننده ها به نیتروژن و آب بی ضرر تبدیل می شود.
کنترل و بهینه سازی: به منظور اطمینان از عملکرد پایدار سیستم SCR و اثر نیترات زدایی کارآمد، سیستم نیاز به کنترل دقیق و بهینه سازی دارد. این شامل نظارت و تنظیم در زمان واقعی دمای گاز دودکش، سرعت جریان، دوز احیاکننده و وضعیت کاتالیزور است.
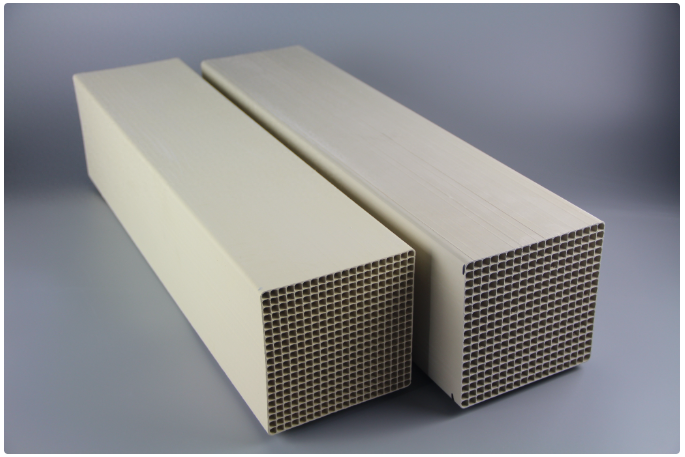
2. نحوه انتخاب کاتالیزور نیترات زدایی
انتخاب مناسب کاتالیزور نیترات زدایی SCR برای اطمینان از عملکرد و کارایی سیستم SCR بسیار مهم است. در اینجا برخی از عوامل کلیدی انتخاب آورده شده است:
فعالیت کاتالیست: فعالیت یک کاتالیزور توانایی آن را در کاتالیز کردن واکنش های کاهش NOx تعیین می کند. کاتالیزورهای بسیار فعال می توانند نرخ تبدیل NOx بالاتری را در دماهای پایین تر به دست آورند. بنابراین هنگام انتخاب کاتالیزورها باید مواد با فعالیت بالا در اولویت قرار گیرند.
انتخاب پذیری: انتخاب پذیری به توانایی یک کاتالیزور برای مهار سایر واکنش های احتمالی (مانند اکسیداسیون آمونیاک) هنگام کاتالیز کردن واکنش های کاهش NOx اشاره دارد. کاتالیزورهای بسیار انتخابی می توانند تشکیل محصولات جانبی را کاهش داده و کارایی نیترات زدایی را بهبود بخشند.
پایداری: پایداری کاتالیست به توانایی آن در حفظ فعالیت و گزینش پذیری خود در طول عملیات طولانی مدت اشاره دارد. کاتالیست هایی با پایداری خوب می توانند در برابر تاثیر مواد مضر موجود در گاز دودکش بر عملکرد آن مقاومت کرده و عمر مفید آن را افزایش دهند.
مقاومت در برابر سمیت: ممکن است موادی در گاز دودکش وجود داشته باشد که برای کاتالیزور سمی هستند، مانند آرسنیک، فلزات قلیایی و غیره. انتخاب یک کاتالیزور با مقاومت سمی خوب می تواند تاثیر این مواد را بر عملکرد کاتالیزور کاهش دهد.
استحکام مکانیکی: کاتالیزور برای مقاومت در برابر آسیب فیزیکی ناشی از جریان گاز دودکش و تغییرات دما نیاز به استحکام مکانیکی خاصی دارد.
مقرون به صرفه بودن: هنگام انتخاب یک کاتالیزور، مقرون به صرفه بودن آن نیز باید در نظر گرفته شود. کاتالیزورهای با کارایی بالا اغلب گران تر هستند، اما در دراز مدت، به دلیل کارایی و پایداری بالا، ممکن است هزینه های عملیاتی کمتری را به همراه داشته باشند.
شهرت تامین کننده و پشتیبانی فنی: انتخاب تامین کننده ای با شهرت خوب و پشتیبانی فنی قوی می تواند کیفیت و پایداری تامین کاتالیزور را تضمین کند و در عین حال پشتیبانی فنی و خدمات پس از فروش لازم را ارائه دهد.